This manual gives you a walk-through on how to use the Stereoisomer Generator Plugin:
Introduction
The Stereoisomer Generator Plugin is able to enumerate all possible stereoisomers of a given compound. The plugin handles both tetrahedral- and double-bond-type stereo centers.
An example for stereoisomer generation output in MarvinView (see Fig. 1) and in MarvinSpace (see Fig. 2) can be found below.
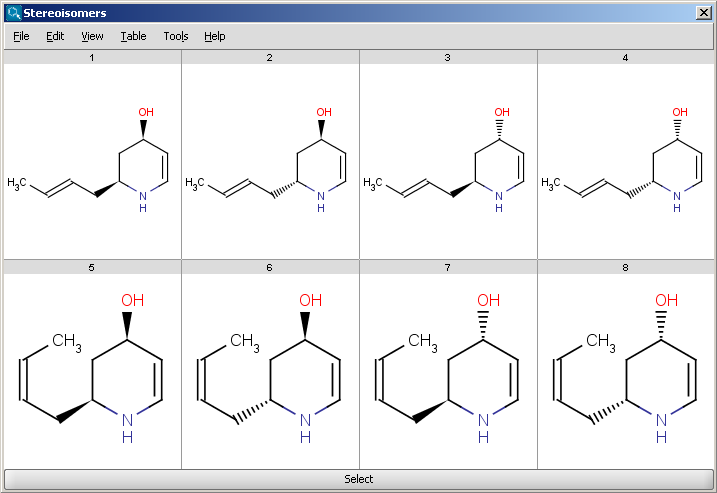
Fig. 1 Generated stereoisomers in MarvinView
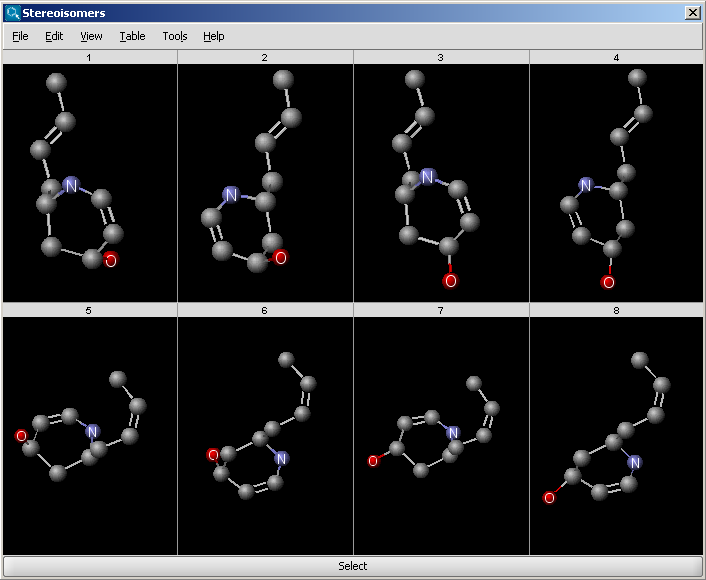
Fig. 2 Generated stereoisomers in MarvinSpace
Options
The panel of the plugin has the following options to set:
- Generate
- Tetrahedral stereo isomers: only the R/S isomers are generated.
- double bond stereo isomers: only E/Z isomers are generated.
- both: both R/S and E/Z isomers are generated.
- Generate all stereoisomers: all isomers are generated
- Generate maximum: sets an upper limit for the number of generated isomers. This option gets enabled if not all isomers are generated.
- Protect tetrahedral stereo centers: if checked, stereocenters already pre-set are not included in the stereoisomer generation.
- Protect double bond stereo: if checked, all double bonds with pre-set stereo information remain intact.
- Filter invalid 3D structures: sterically restricted isomers are discarded.
- Display in 3D: results are displayed in a 3D viewer. By default the results are shown in a 2D panel, one for each generated isomer. This option is enables only if 3D filtering is enabled.
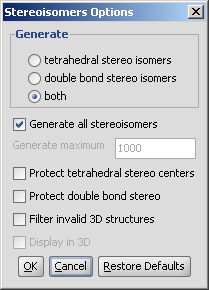
Fig. 3 Options window for stereoisomer calculation
References
- Smith, M. B.; March, J. Advanced Organic Chemistry, 5th ed., Wiley Interscience, New York, 2001; pp 1218-1223. ISBN 0471585890