|
|
|
|
|
|
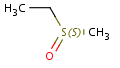
The dimension of a molecule can be interpreted topologically, based on the connections of the consisting atoms, or spatially, based on the Cartesian coordinates of them. In this section the notion of dimension is used in spatial sense.
Molecules with same connectivity but different spatial arrangement are called stereoisomers.
Stereoisomer types:
|
|
|
|
|
|
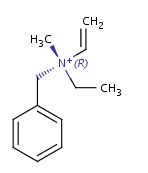
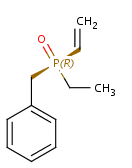
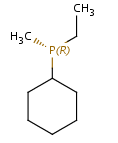
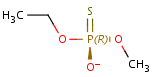
In general, single bonds are rotatable, but double bonds are not. If the substituents on each side of the double bond are different, then two diastereomers of the molecule can be distinguished based on the orientation of the ligands. Two substituents located on the same side of the double bond are referred to as cis isomer, otherwise, if the two substituents are located on the opposite side it is referred to as trans isomer.
|
|
|
trans-1,2-dichloroethene |
cis-1,2-dichloroethene |
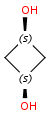
Alicyclic compounds can also display cis-trans isomerism. In this case a single bond becomes non rotatable due to constrain of a cycle. However, in these cases we use tetrahedral stereochemistry.
|
|
|
(1R,2S)-1,2-dichlorocyclohexane |
(1S,2S)-1,2- |
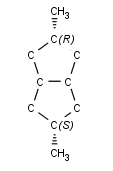
The cis/trans system for naming isomers is not effective if more than two different substituents are attached to the double bond. In this case, following the Cahn-Ingold-Prelog priority rules, a priority is assigned to each substituent on a double bond. If the two groups of higher priority are on opposite sides of the double bond (trans arrangement), then the E configuration is assigned to the bond. If the two groups of higher priority are on the same side of the double bond (cis arrangement), than the Z configuration is assigned to it.
|
|
2E-2,3-dichlorobut-2-ene |
2Z-2,3-dichlorobut-2-ene |
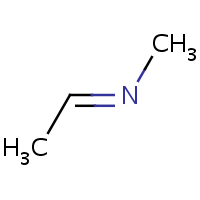
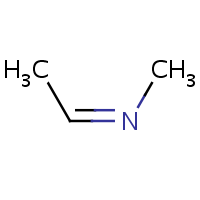
E/Z stereochemistry of the nitrogen atom is also supported:
|
|
| (E)-ethylidene(methyl)amine | (Z)-ethylidene(methyl)amine |
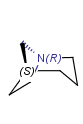
An atom in the molecule around which the ligands are arranged so that interchange of two ligands leads to stereoisomer is called stereocenter or stereogenic center. Chirality appears in stereoisomerism which is due to tetrahedral stereogenic centers. These centers can have point chirality. The ligands of the chiral center are assigned a priority based on the Cahn-Ingold-Prelog priority rules. Each chiral center is then labeled by R or S based on the orientation of the assigned numbers. The center is oriented so that the lowest-priority is pointed away from the viewer. If the priority of the remaining three substituents decreases clockwise, it is labeled R, otherwise, if it decreases counter clockwise, it is S.
|
|
R-bromo(chloro)iodomethane |
S-bromo(chloro)iodomethane |
Pseudo-asymmetric atoms which are invariant on reflection in a mirror
are labeled by r/s descriptors:
Explained in Wikipedia: Cahn-Ingold-Prelog priority rules.
Hindered rotation around single bonds where the steric strain barrier to rotation is high enough to allow the isolation of the conformers resulting in atrop stereoisomerism.
If two stereoactive atoms (atoms with at least three different ligands) are connected by an even numbered chain of rigid parts then axial stereo information can be defined on the ligands of the stereactive atoms. These ligands are the ones which are not in the chain of the rigid part.
The following substructures are considered as rigid parts:
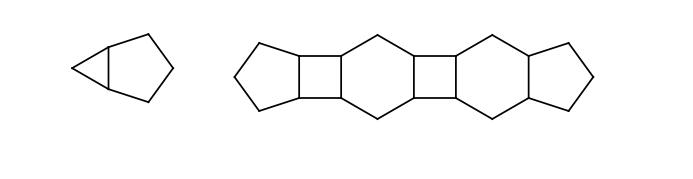
You may find more information concerning stereochemistry in the query guide or in the developer guide.
The relative position of ligands on a chiral atom is marked with wedge bonds: up (solid), down (hatched), up or down (wiggly). Having wedge bonds at chiral atoms with the chiral flag on the entire structure implies that a single isomer is present. The absolute configuration (R or S) is known for all chiral centers that are marked with wedge bonds.
Non-stereo bond to atom at stereogenic centers implies that no information is known about the configuration of a stereogenic center. It could be either of two stereoisomers, or a mixture of the two.
The existence of wedge bonds at chiral atoms without chiral flag
on the entire structure has two meanings depending on the file format
used.
MDL file types (mol, sdf ...):
The structure is a racemic mixture of the two enantiomers.
Daylight file types (smiles, smarts):
Wedges mean absolute stereo configuration, the structure represents a
single enantiomer.
The positions of the double bond ligands already define the stereo configuration of the double bond (cis or trans). Special query double bond types allow us to specify cis or trans, not trans or not cis isomers.
Works in MDL molecule formats: mol, rgf, sdf, rxn etc... and in ChemAxon Extended SMILES format: cxsmiles.
Enhanced stereochemical representation introduces three types of identifiers that can be attached to a stereogenic center. A stereochemical group label is composed from an identifier and a group number. Each stereogenic center marked with wedge bonds belongs to one (and only one) stereochemical group. Grouping allows us to specify relative relationships among stereogenic centers.
Stereochemical group types:
- ABS
Stereogenic center where the absolute configuration is known.- OR
Stereogenic center where the relative configuration is known, but the absolute configuration is not known. The structure represents one stereoisomer that is either the structure as drawn (R,S) OR the epimer in which the stereogenic centers have the opposite configuration (S,R).- AND
Mixture of stereoisomers. It can be a pair of enantiomers or all the diastereomers.
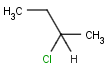
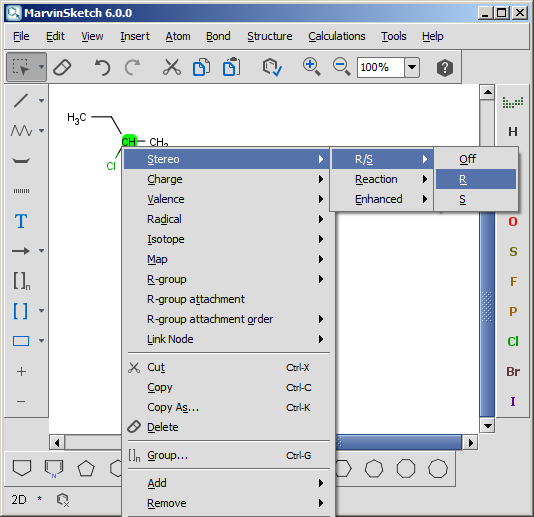
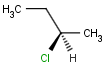
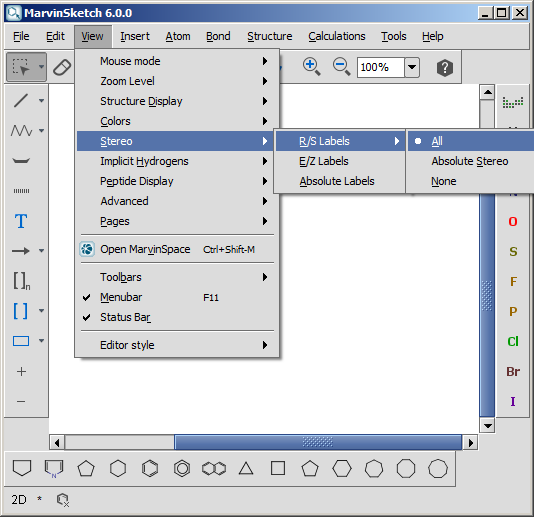
| [1] | http://accelrys.com/products/informatics/cheminformatics/ctfile-formats/no-fee.php |